Molecular Orbitals for First- and Second-Row Diatomic Molecules

This Demonstration considers the molecular orbitals for the diatomic molecules 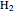 through
through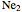 .
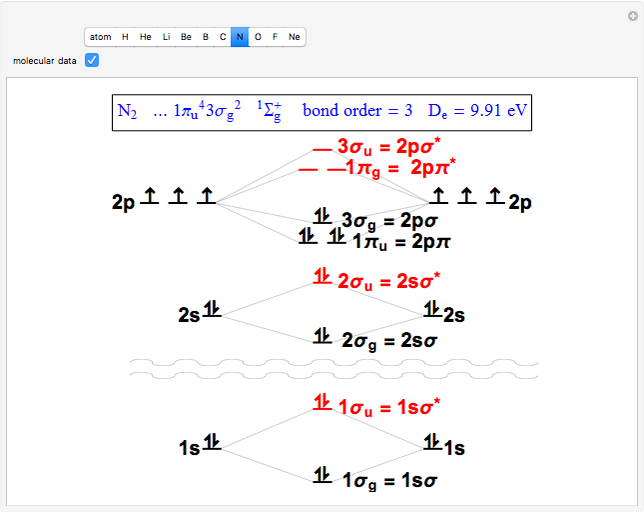
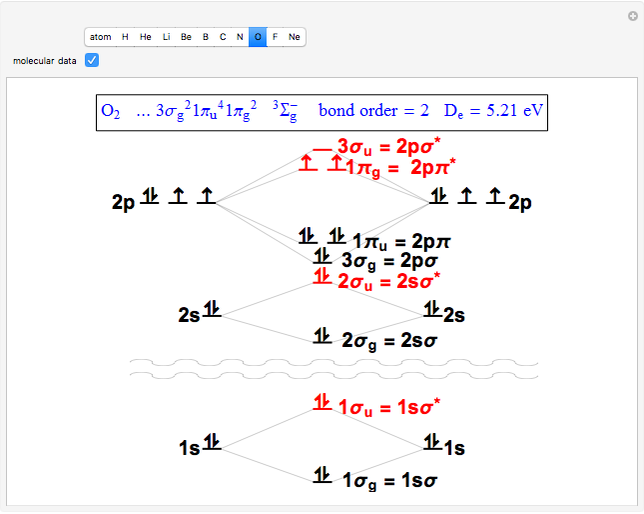
The conceptual relationship to the constituent atomic orbitals is shown
in a schematic energy diagram. Antibonding MOs are shown in red. The
bond order of a molecule is defined as the number of electrons occupying
bonding MOs minus the number occupying antibonding MOs, all divided by
2, to coincide with the conventional chemical definition of single,
double, and triple bonds. The molecules
.
The conceptual relationship to the constituent atomic orbitals is shown
in a schematic energy diagram. Antibonding MOs are shown in red. The
bond order of a molecule is defined as the number of electrons occupying
bonding MOs minus the number occupying antibonding MOs, all divided by
2, to coincide with the conventional chemical definition of single,
double, and triple bonds. The molecules 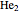 and
and 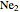 have bond orders equal to 0, and exist only as weakly-associated van der Waals dimers.
have bond orders equal to 0, and exist only as weakly-associated van der Waals dimers. 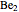 also has bond order 0, but forms a weak chemical bond attributed to some 2s-2p
hybridization. The "molecular data" checkbox brings up the ground state
electron configuration and spectroscopic designation for each diatomic.
The dissociation energy
also has bond order 0, but forms a weak chemical bond attributed to some 2s-2p
hybridization. The "molecular data" checkbox brings up the ground state
electron configuration and spectroscopic designation for each diatomic.
The dissociation energy  is also given, showing an expected increase with bond order.
is also given, showing an expected increase with bond order.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: the simplest diatomic molecule 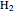 , with bond order 1; the hydrogen molecule ion
, with bond order 1; the hydrogen molecule ion 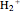 is also stable with a formal bond order of 1/2 and
is also stable with a formal bond order of 1/2 and  =2.79 eV
=2.79 eV
Snapshot 2: the nitrogen molecule has an excess of six occupied bonding over antibonding orbitals, thus a bond order of 3, a triple bond
Snapshot 3: diatomic oxygen is predicted to be paramagnetic in a triplet electronic state, a major triumph of molecular-orbital theory
Reference: S. M. Blinder, Chap. 11, Introduction to Quantum Mechanics, Amsterdam: Academic Press, 2004.
Permanent Citation
https://demonstrations.wolfram.com/MolecularOrbitalsForFirstAndSecondRowDiatomicMolecules/


No comments:
Post a Comment