equilibrium separation R e |
exponential parameter a |

For both classical and quantum systems with any number of particles interacting by inverse-square forces ( potentials)—electrostatic or gravitational—the average potential and
kinetic energies obey the virial theorem. This states that the average
of the potential energy equals the negative of half the average of the
kinetic energy:
potentials)—electrostatic or gravitational—the average potential and
kinetic energies obey the virial theorem. This states that the average
of the potential energy equals the negative of half the average of the
kinetic energy:  . Since the total energy is given by
. Since the total energy is given by  ,
,  , while
, while  In classical mechanics,
In classical mechanics,  and
and  pertain to the time averages of these quantities per period of the
motion, while in quantum mechanics they mean the expectation values of
the potential and kinetic energy operators. For example, in the ground
state of the hydrogen atom, with
pertain to the time averages of these quantities per period of the
motion, while in quantum mechanics they mean the expectation values of
the potential and kinetic energy operators. For example, in the ground
state of the hydrogen atom, with  eV, we have
eV, we have  eV and
eV and  eV.
eV.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
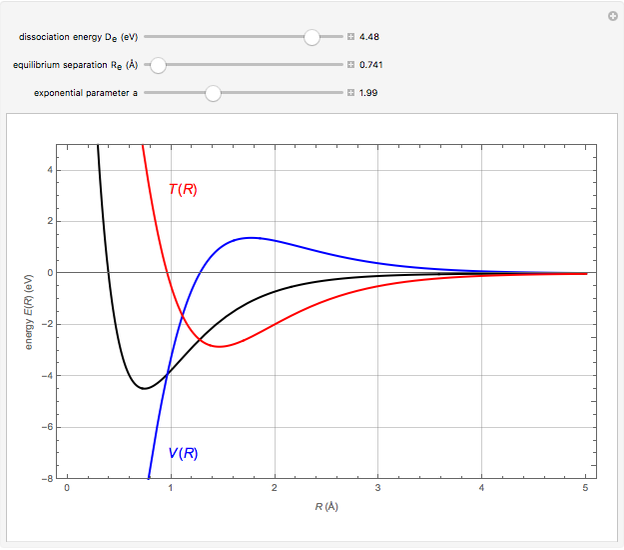
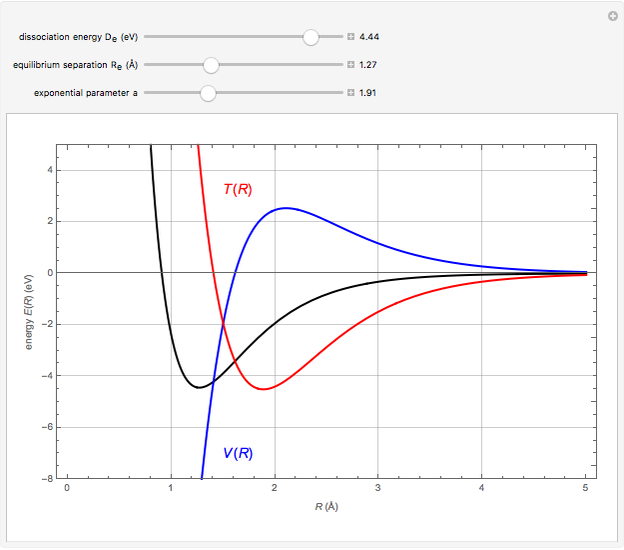
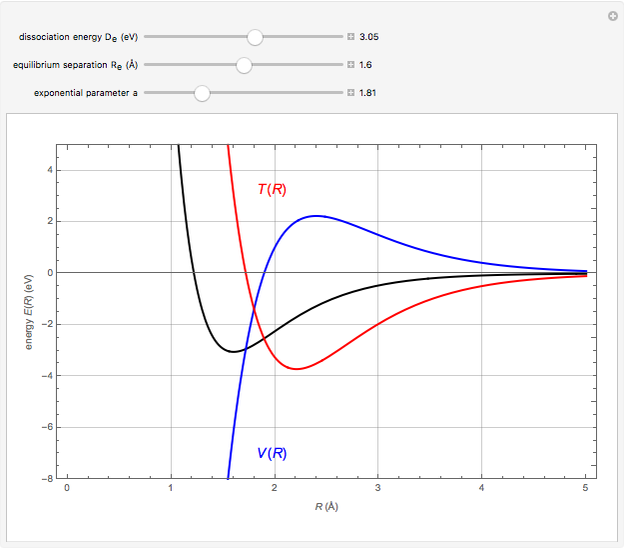
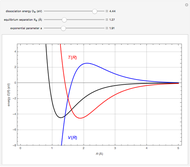
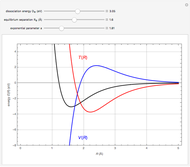
Snapshot 1:  molecule; Snapshot 2: HCl molecule; Snapshot 3: HI molecule
molecule; Snapshot 2: HCl molecule; Snapshot 3: HI molecule
References:
[1] J. C. Slater, "The Virial and Molecular Structure," Journal of Chemical Physics, 1(10), 1933 pp. 687-691.
[2] J. P. Lowe and K. A. Peterson, Quantum Chemistry 3rd ed., Amsterdam: Elsevier, Academic Press, 2006 p. 628.
Permanent Citation
https://demonstrations.wolfram.com/VirialTheoremForDiatomicMolecules/

No comments:
Post a Comment